Background
Sub-Saharan African countries are the hardest hit by sickle cell disease (SCD). Several studies have demonstrated the remarkable efficacy of hydroxyurea (HU) in African sickle-cell populations. As a result, many specialists recommend widespread use of this drug in Africa to reduce the disease's high morbidity/mortality. Nevertheless, in those countries health insurance mechanisms do not exist or and many families affected by SCD do not have enough income to pay for the care. In addition to cost, frequent supply chain disruptions lead to low drugs availability.
The cost of basic treatments for SCD and their actual availability in different African countries is not yet known. Yet this information is essential for guiding health policies or the action of non-governmental organizations, and for planning the cost of assistance that could be provided to these patients.
The aim of this study was to determine the availability, frequency of supply disruptions and cost of basic treatments for SCDin sub-Saharan African countries.
Methods
A cross-sectional online international survey was carried out from 24 March to 08 May 2023 among health professionals from French-speaking sub-Saharan African countries following SCD patients and registered in the database of the non-governmental organisation (NGO) Drep-Afrique. Participants completed questions covering the availability (on a scale from 0 (drug not available) to 10 (no availability concern) and the cost of basic drugs in their centres: HU, penicillin V, folic acid, morphine and vaccines.
Results
120 health professionals, mainly physicians (91%), from 13 French-speaking Sub-Saharan African countries took part in this study. 58% of the participants worked in SCD referral centres and 36% in university hospitals. 91,000 homozygous SCD patients were reported as being followed in their centres.
The median availability score for hydroxyurea and morphine were respectively 5 (IQR 3-8) and 5 (IQR 2-7), compared with 10 (IQR 10-10) for folic acid, 8 (IQR 4-10) for penicillin V, 3 (IQR 1-8) for anti-pneumococcal and 5 (IQR 2-8) for anti-meningococcal vaccines.
Frequent supply chain disruptions were declared: 78% for HU, 77% for anti-pneumococcal vaccine, 66% for morphine, 45 % for Penicillin V, while they were rare for folic acid (8%).
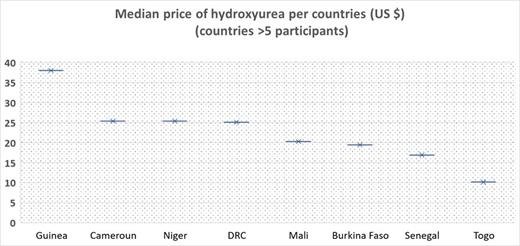
The median price of HU for one month's treatment of a 30 kg child (mean dose 20 mg/kg/day) was $19.3 (IQR 13.5- 25.3) and varied greatly from country to country (from $38 in Guinea to $10.1 in Togo (p=0.0002) (figure 1).
The median price of pneumococcal vaccine was $35.3 (IQR 16.9- 58.7), and thant of meningococcal vaccine $31.4 (IQR 11- 57.5), also with significant differences between countries. The cost of pneumococcal vaccine was 1.6 times higher in centres where the supply chain disrupted than when there is no disruption (p=0.02).
Conclusion
This study shows that the cost of HU varies from one country to another in French-speaking Africa. Although not very high, it remains unaffordable for the most patients' families. For example, in 2023, the median monthly salary is 238 $ in the Democratic Republic of Congo (the country with the highest prevalence of SCD in the world with Nigeria), compared with a median monthly cost of HU of 25 $ in this country for a child in our study. The high cost of vaccines, beyond the first years of life when a free national program is available in most of African countries, is also a problem. In addition to cost, availability and frequent supply disruptions prevent regular intake of hydroxyurea and, to a lesser extent, of penicillin V. International solidarity and the governments of these countries should work to bring down the cost of hydroxyurea and improve the distribution circuit for these essential medicines.
Disclosures
de Montalembert:Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: ASH meeting 2022 support; Novartis: Membership on an entity's Board of Directors or advisory committees. Coulibaly:Fresenius Kabi: Current Employment. Arlet:Addmedica: Research Funding; Novartis: Research Funding; Vertex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal